Information For
Parelaphostrongylus Tenuis (Meningeal Worm)
Prevention & Diagnosis
Treatments
"Brain Worm" (Meningeal Worm) Infestation in Llamas and
Alpacas
(March 21,
2013 Knoxville, TN)
David E Anderson, DVM, MS, DACVS
Department of Large Animal Clinical Sciences
College of Veterinary Medicine
University of Tennessee
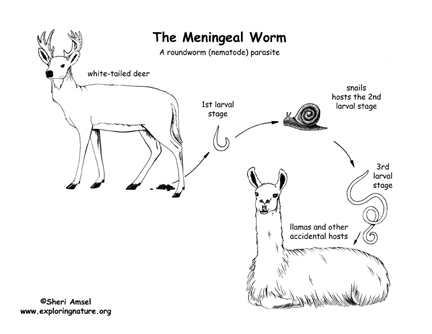
The meningeal worm (Parelaphostrongylus tenuis), also
known as the deer worm or meningeal deer worm, frequently infects llamas
and alpacas. Aberrant migration of the meningeal worm in susceptible
hosts such as llamas and alpacas
causes damage to the central nervous system and may result in death.
Identification and
Life Cycle
The meningeal worm is a nematode parasite belonging to
the family Protostrongylidae. The definitive host is the whitetailed
deer (Odocoileus virginianus) prevalent throughout much of eastern North
America.1 Adult
meningeal worms reside in the meninges of whitetailed deer and rarely
cause clinical signs of disease.1,2
Adult worms lay eggs in the meninges of whitetailed
deer. The eggs then pass into the venous circulation and travel to the
lungs where they hatch into firststage larvae (L1). The L1 are coughed
up, swallowed, and passed in the feces of infected deer. Larvae then
invade or are ingested by snails or slugs (terrestrial
gastropods). Snails and slugs serve as intermediate hosts in which the
first stage larvae develop into infective third stage larvae (L3) over a
period of 34 weeks.13 Infected
snails or slugs are then ingested by susceptible aberrant hosts such as
llamas, alpacas, goats, sheep, moose, wapiti, caribou, blacktailed deer,
and red deer1, and the L3
are released in the digestive tract. Infective third stage larvae
migrate to the spinal cord and continue to migrate aimlessly within the
central nervous system causing neurologic disease.13
In the definitive host, the whitetailed deer, the
infected snails or slugs are ingested and the L3 are released in the
abomasum. The L3 then migrate to the spinal cord via the spinal nerves
over the next 10 days. The larvae mature in the dorsal horns of the gray
matter of the spinal cord for 2030 days. Adult meningeal worms migrate
to the subdural space, then to the brain through the dura mater and
cranial venous sinuses.2 The
prepatent period in deer is 82-92 days.2,4
Many snails and
slugs prefer a moist or wet environment for survival. Consequently,
lowlying and wet, poorly drained fields provide an increased risk of
exposure to snails and slugs.3 However,
exposure risk is not limited to wet climates since dry-land snails and
slugs may serve as intermediate hosts. Snails and slugs feed on organic
matter, leaf litter, and vegetation. Survival of L3 outside the
intermediate host is believed to be shortlived unless water is
available. Repeated freezing or desiccation has been shown to decrease
survival of the infective L3.2 Therefore,
the risk of exposure to llamas and alpacas is lowest when there are
prolonged periods of dryheat or deep freezes.
Clinical Disease
Once the aberrant
host is infected, clinical disease begins 45-53 days later as
demonstrated by experimental inoculation. 4 Clinical
neurologic disease is the result of tissue destruction and inflammation
caused by randomly migrating larvae. Thus, the clinical signs observed
depend upon the location of the migrating larvae.3
Most commonly, clinical signs reflect asymmetrical, focal
spinal cord lesions.4 These
include hypermetria,2,5 ataxia,1,2,5,6 stiffness,1,2 muscular
weakness,2,5,6 posterior
paresis,2,6 paralysis,1,2 head
tilt,2 arching
neck,2circling,1,2 blindness,1,2 gradual
weight loss,2 apparent
depression, seizures, and death.2 Clinical
signs generally begin in the hind limbs and progress to the front limbs.2,4 The
course of disease may be acute to chronic, ranging from death within
days to ataxia which lasts months to years.2 In
our experience, clinical sings of meningeal worm infection are
exacerbated during summer months because heat stress develops with
prolonged periods of recumbency.
Prevention
Prevention of meningeal worm infection may be
difficult. Ideally, llamas should not graze the same pasture as
whitetailed deer.2,9 However,
in many areas of the United States, it is not feasible to separate the
two populations.
Tools to minimizing
risk and minimize use of drugs:
-
Placing a deerproof fence may offer some protection,
but many fences do not present a sufficient barrier to prevent
movement of deer. A true deer proof fence is 12 feet high and of woven
wire not high tensile fencing.
-
Eliminate
organic matter. Snails and slugs prefer dark, damp areas. Thus,
these pests will accumulate around leaves, buildings, wood piles,
compost, etc. Keep areas around pasture and barns clear.
-
Thick ground
cover can be removed to expose the environment to fluctuations
in temperature, and vegetationfree buffer zones (i.e. gravel,
limestone) can be placed around fence lines to reduce migration of
snails and slugs into the pasture.
-
Fowl (e.g.
Guinea hens) may be used to help decrease pasture contamination with
snails and slugs. Molluscicides may be considered to destroy
snails and slugs which serve as intermediate hosts, thereby
interrupting the life cycle of the meningeal worm and preventing
infection in aberrant hosts. However, molluscicides can not be used in
heavy amounts or frequently without creating a build-up of toxins in
the environment. Drainage should be established in low lying areas and
access to swampy areas may be
restricted by fencing off these areas. These compounds present
a potential environmental risk from contamination of ground water and
may be toxic if consumed by camelids or other animals.
-
The slugs and snail that transmit meningeal worm larvae
include a large array of arboreal slugs not aquatics. Thus, pastures
with leaf piles, etc are equally at risk as pasture with ponds or
streams.
-
Geography: If
you live in an area where hard freezes (Ohio from Dec-March) and dry
summers are severe, transmission is at a minimal risk during those
periods. In spring, slug and snail contamination is minimal as the
over wintering is harsh. In these geographic areas, more than 85% of
transmission occurs during Sept-Dec and we can selectively target long
acting avermectin drug prevention during those times. In
geographic areas of high deer density and established meningeal worm
in the populations, these seasonal effects are unlikely to be
relevant.
Using Drugs to Plug
the Holes:
Based on 30 years of field experiences and our clinical
and pharmacologic research,
prophylactic treatment against migrating larvae may be achieved by
administration of ivermectin (0.3 mg/kg) every 30 days during the high
risk periods or throughout the year in regions which have mild summers
and winters. Anthelmintic resistance is unlikely to become a
problem in the meningeal worm because these infections do not become
patent in llamas and alpacas.Meningeal worm infection has occurred in
some herds that maintain vigilant prophylaxis. These "breaks" innthelmintic, accidental failure to administer the
anthelmintic, or extremely high environmental contamination. Employing
environmental management tools will reduce risk and help maintain a
healthy meningeal worm free herd.
On the Horizon
Researchers are studying the use of a vaccine developed
against meningeal worm. Hopefully this will enable us to protect the
animals, but limit use of drugs such as ivermectin. The constant use of
ivermectin over the past 20 years is leading to build up of extremely
dangerous intestinal parasites in herds and will eventually be more
dangerous that meningeal worms.
REFERENCES
1. Fowler, ME:
Medicine and Surgery of South American Camelids. Ames, Iowa:Iowa State
University Press 161162; 1989.
2. Pugh, DG et
al: Clinical parelaphostrongylosis in llamas. Compendium on Cont. Ed.
for Pract. Vet. 17:600606;1995.
3. Smith, MC et
al: Goat Medicine. Philadelphia:Lea & Febiger 150; 1994.
4. Rickard, LG et
al: Experimentally induced meningeal worm (Parelaphostrongylus tenuis)
infection in the llama (Lama glama): Clinical evaluation and
implications for parasite translocation. J Zoo Wildl Med 25:390402;
1994.
5. Foreyt, WI et
al: Experimental infections of two llamas with the meningeal worm (Parelaphostrongylus
tenuis ). J Zoo Wildl Med 23:339344;1991.
6. Baumgartner, W
et al: Parelaphostrongylosis in llamas. J Am Vet Med Assoc
187:12431245;1985.
7. Welles, EG et
al: Composition of cerebrospinal fluid in healthy adult llamas. Am J Vet
Res 55:10751079;1994.
8. Dew, TL et al:
Parasitespecific immunoglobulin in the serum and cerebrospinal fluid of
whitetailed deer (Odocoileus virginianus) and goats (Capra hircus) with
experimentally induced parelaphostrongylosis. J Zoo Wildl Med
23:281287;1992.
9. Rickard, LG.
Parasites. Vet Clin North Am Food Anim Pract 10:239247;1994.
10. MacDiarmid,
SC. Clinical pharmacology of the female reproductive tract: manipulation
of parturition, its sequeslae and infections. Clinical Pharmacology and
Therapeutics Proceedings 71:21-45;1984.
11. Kopcha, M et
al: Cerebrospinal nematodiasis in a goat herd. J Am Vet Med
Assoc 194:14391442;1989.
|
Parelaphostrongylus Tenuis (Meningeal Worm)
Infection in Llamas and Alpacas
David E Anderson, DVM, MS, Associate Professor
Department of Veterinary Clinical Sciences,
College of Veterinary Medicine,
The Ohio State University,
Columbus, Ohio 43210.
The meningeal worm (Parelaphostrongylus tenuis), also known as the deer worm
or meningeal deer worm, frequently infects llamas and alpacas. Aberrant
migration of the meningeal worm in susceptible hosts such as llamas and alpacas
causes damage to the central nervous system and may result in death.
Identification and Life Cycle
The meningeal worm is a nematode parasite belonging to the family
Protostrongylidae. The definitive host is the white-tailed deer (Odocoileus
virginianus) prevalent throughout much of eastern North America.1
Adult meningeal worms reside in the meninges of white-tailed deer and rarely
cause clinical signs of disease.1,2
Adult worms lay eggs in the meninges of white-tailed deer. The eggs then pass
into the venous circulation and travel to the lungs where they hatch into
first-stage larvae (L1). The L1 are coughed up, swallowed, and passed in the
feces of infected deer. Larvae then invade or are ingested by snails or slugs
(terrestrial gastropods). Snails and slugs serve as intermediate hosts in which
the first stage larvae develop into infective third stage larvae (L3) over a
period of 3-4 weeks.1-3
Infected snails or slugs are then ingested by susceptible aberrant hosts such
as llamas, alpacas, goats, sheep, moose, wapiti, caribou, black-tailed deer, and
red deer1, and the L3 are released in the digestive tract. Infective third stage
larvae migrate to the spinal cord and continue to migrate aimlessly within the
central nervous system causing neurologic disease.1-3
In the definitive host, the white-tailed deer, the infected snails or slugs
are ingested and the L3 are released in the abomasum. The L3 then migrate to the
spinal cord via the spinal nerves over the next 10 days. The larvae mature in
the dorsal horns of the gray matter of the spinal cord for 20-30 days. Adult
meningeal worms migrate to the subdural space, then to the brain through the
dura mater and cranial venous sinuses.2 The prepatent period in deer
is 82-92 days.2,4
Many snails and slugs prefer a moist or wet environment for survival.
Consequently, low-lying and wet, poorly drained fields provide an increased risk
of exposure to snails and slugs.3 However, exposure risk is not
limited to wet climates since dry-land snails and slugs may serve as
intermediate hosts. Snails and slugs feed on organic matter, leaf litter, and
vegetation. Survival of L3 outside the intermediate host is believed to be
short-lived unless water is available. Repeated freezing or desiccation has been
shown to decrease survival of the infective L3.2 Therefore, the risk of exposure
to llamas and alpacas is lowest when there are prolonged periods of dry-heat or
deep freezes.
Clinical Disease
Once the aberrant host is infected, clinical disease begins 45-53 days later
as demonstrated by experimental inoculation. 4 Clinical neurologic
disease is the result of tissue destruction and inflammation caused by randomly
migrating larvae. Thus, the clinical signs observed depend upon the location of
the migrating larvae.3
Most commonly, clinical signs reflect asymmetrical, focal spinal cord
lesions.4 These include hypermetria,2,5 ataxia,1,2,5,6
stiffness,1,2 muscular weakness,2,5,6 posterior paresis,2,6
paralysis,1,2 head tilt,2 arching neck,2
circling,1,2 blindness,1,2 gradual weight loss,2
apparent depression, seizures, and death.2 Clinical signs generally
begin in the hind limbs and progress to the front limbs.2,4 The
course of disease may be acute to chronic, ranging from death within days to
ataxia which lasts months to years.2 In our experience, clinical
sings of meningeal worm infection are exacerbated during summer months because
heat stress develops with prolonged periods of recumbency.
Differential Diagnoses
Clinical signs suggestive of meningeal worm infection are non-specific and
may affect the spinal cord or brain. Clinical signs of spinal cord lesions
include weakness, ataxia, gait abnormalities, lameness, proprioceptive deficits,
paresis, and paralysis. Differential diagnoses (Table 1) for camelids with these
clinical signs may include vertebral body subluxation or fracture, vertebral
body abscessation, trauma, spinal neoplasia, degenerative myelopathy, metabolic
diseases such as copper deficiency in neonates, listeriosis, heat stress, and
tick paralysis. Clinical signs of intracranial disease include ataxia, abnormal
mentation (dementia, stupor, coma), visual abnormalities, circling, falling or
rolling, weakness, delayed postural reactions, incoordination, head tilt,
altered head and neck position, nystagmus, strabismus, and seizures.
Differential diagnoses for camelids with these signs may include neoplasia,
trauma, hydrocephalus or other congenital defects, cerebral abscessation,
listeriosis, otitis interna, and polioencephalomalacia. Electrolyte imbalances
such as hypocalcemia, hypomagnesemia, and hypoglycemia, ketosis, and dietary
deficiencies such as copper, vitamin A, vitamin E, selenium, calcium, magnesium,
potassium, and phosphorus may each present neurologic signs of disease. In
addition, consider toxicoses such as lead poisoning, ingestion of poisonous
plants, and salt poisoning. Rabies encephalitis may present with a variety of
neurologic signs and should be considered in any neurologic case. These
differential diagnoses must be ruled out prior to making a presumptive diagnosis
of meningeal worm infection. Although consistent clinical signs and CSF
eosinophilia are highly suggestive of meningeal worm infection, antemortem
diagnosis of aberrant Parelaphostrongylus tenuis migration is often a diagnosis
of exclusion.
Diagnostic Testing
To thoroughly evaluate the patient, collect a database of information which
includes the signalment, history including the onset and progression of clinical
signs, complete blood count, and serum chemistry profile. Additional diagnostic
testing include vertebral radiography, cerebrospinal fluid (CSF) analysis and
culture, CSF creatine kinase activity, and plasma fibrinogen concentration. In
select cases, advanced diagnostic testing such as myelography, X-ray computed
tomography (CT), magnetic resonance imaging (MRI), or electromyography (EMG) may
be indicated to rule-out other causes of spinal or intra-cranial disease.
Diagnosis of Meningeal Worm
A presumptive diagnosis is based upon clinical signs consistent with
meningeal worm infection, history of exposure to areas inhabited by white-tailed
deer, and response to treatment.2,6 No consistent abnormalities in
CSF total protein or glucose concentrations, AST or CK activities were
identified in llamas experimentally infected with P. tenuis4 (Table 2). The only
consistent abnormality was a shift in nucleated cell count from predominantly
lymphocytes and monocytes to eosinophils over the course of infection.4,9
The presence of clinical signs and CSF eosinophilia may be used to make a
tentative diagnosis of P. tenuis infection in llamas.2,4,6,7
Cerebrospinal fluid analysis may show eosinophilia, but absence of eosinophilia
on CSF analysis does not rule out the diagnosis of meningeal worm infection.2,5,6
However, CSF eosinophilia in llamas has been most consistently reported in cases
of clinical parelaphostrongylosis.2,6,9 Hematologic samples may show
peripheral eosinophilia but frequently show no abnormalities.2,6
One study showed a significant P. tenuis - specific serum antibody response
in goats experimentally infected with 50 P. tenuis L3.8 Serum
antibody titers were highest 8 weeks after infection. Results of this study
suggest that a serum enzyme-linked immunosorbent assay (ELISA) using antigens of
adult P. tenuis would be beneficial in the diagnosis of clinical
parelaphostrongylosis in goats.2 Modification of this test may show
promise in detecting parelaphostrongylosis in llamas.2,9
Definitive diagnosis of meningeal worm infection is made at necropsy. A
confirmed diagnosis requires microscopic demonstration of the larvae within the
brain or spinal cord.2,9 Microscopic examination of brain and spinal
cord tissue may also show linear paths of tissue damage or inflammation
suggestive of migrating tracts made by the larvae.6
Therapy
Treatment of meningeal worm infection is most successful when instituted
early in the course of disease. A treatment regime (Table 3) which has proven
successful at the Ohio State University involves fenbendazole (20 to 50 mg/kg
body weight, PO, q24h for 5 days) and flunixin meglumine (1 mg/kg, IV, IM, or
SC, q12h for 5 days) or dexamethasone in non-pregnant females and males (0.1
mg/kg, IV, IM, or SC, q24h for 3 days). DMSO (1g/kg given in 500 ml of 5%
dextrose solution, IV, q24h) given to effect is useful in some cases but may
cause severe appetite suppression. Discontinue DMSO if inappetance or anorexia
occurs. Vitamin E, selenium, Vitamins B-complex, and Vitamin A are useful to
assist healing of neural tissues.
Dexamethasone should not be administered to pregnant females because this
drug may induce abortion. Alternatively, we have used prednisolone sodium
succinate (0.5-1.0 mg/kg, IV, IM, or SC, q12h) for no more than three days in
pregnant females without subsequent abortion. Prednisolone sodium succinate may
have a reduced risk of abortion compared to dexamethasone because it lacks a
carbon-16 substitution. Corticosteroids lacking a C-16 substitution may not
cross the blood-placental barrier and large doses for prolonged periods of time
may be required to terminate pregnancy.10
Ivermectin is most effective against larval stages prior to their entrance
into the spinal cord, since it does not readily cross the blood-brain barrier.1,2,11
However, damage to nervous system tissues during larval migration may alter the
blood-brain barrier. Although no clinical problem has been identified to date,
we have been concerned for the possibility of ivermectin toxicity in these
cases. The antiinflammatory drugs are critical to reduce the inflammation
associated with the presence of the migrating larvae and the subsequent
inflammatory response to the killed larvae. Use of antiinflammatory drugs is
important to prevent the clinical signs from becoming more severe after
instituting treatment.
In addition to drug therapy, supportive care and physical therapy are
essential to aiding recovery. Using slings to support llamas that are unable to
stand and performing physical therapy for muscles are beneficial. We also have
used hydrofloatation therapy to facilitate recovery after prolonged recumbency
(Figure 1). A great deal of perseverance is required to care for severely
affected llamas; recovery may take several weeks to months to years.2
Prognosis
Prognosis for survival depends upon how severe the clinical signs become. In
our experience, llamas that are unable to stand have a poor prognosis (10-20%
recovery); llamas that are able to stand unaided have a fair to good prognosis
(75-85% recovery). Llamas that survive clinical disease do not seem to develop
patent infections and are unlikely to pose a health risk to other animals.2,3
Many animals suffer permanent neurologic deficits but may remain productive
members of the herd for breeding and pets.
Prevention
Prevention of meningeal worm infection may be difficult. Ideally, llamas
should not graze the same pasture as white-tailed deer.2,9 However,
in many areas of the United States, it is not feasible to separate the two
populations. Placing a deer-proof fence may offer some protection, but many
fences do not present a sufficient barrier to prevent movement of deer.1,2
Additionally, thick ground cover can be removed to expose the environment to
fluctuations in temperature, and vegetation-free buffer zones (i.e. gravel,
limestone) can be placed around fencelines to reduce migration of snails and
slugs into the pasture.2,9 Molluscicides may be considered to destroy
snails and slugs which serve as intermediate hosts, thereby interrupting the
life cycle of the meningeal worm and preventing infection in aberrant hosts.1
Drainage should be established in low lying areas and access to swampy areas may
be restricted by fencing off these areas. These compounds present a potential
environmental risk from contamination of ground water and may be toxic if
consumed by camelids or other animals.
Prophylactic treatment against migrating larvae may be achieved
administration of ivermectin (0.2 mg/kg) every 30 to 45 days during the high
risk periods or throughout the year regions which have mild summers and winters.
Anthelmintic resistance is unlikely to become a problem in the meningeal worm
because these infections do not become patent.2 However, meningeal
worm infection has occurred in some herds that maintain vigilant prophylaxis.
These "breaks" in prevention of the larval migration may have been
caused by insufficient dosing of anthelmintic, accidental failure to administer
the anthelmintic, or some unknown mechanism.
Conclusion
Meningeal worm infection may be severely debilitating and potentially fatal,
but infection can be effectively prevented. Routine dewormings every 4-6 weeks,
minimized cohabitation with white-tailed deer, and a clean, dry environment
unfavorable for the growth of snails and slugs will considerably reduce the
herd’s risk of infection with the meningeal worm.
References
- Fowler, ME: Medicine and
Surgery of South American Camelids. Ames, Iowa:Iowa State University Press
161-162; 1989.
- Pugh, DG et al: Clinical
parelaphostrongylosis in llamas. Compendium on Cont. Ed. for Pract. Vet.
17:600-606;1995.
- Smith, MC et al: Goat
Medicine. Philadelphia:Lea & Febiger 150; 1994.
- Rickard, LG et al:
Experimentally induced meningeal worm (Parelaphostrongylus tenuis) infection in
the llama (Lama glama): Clinical evaluation and implications for parasite
translocation. J Zoo Wildl Med 25:390-402; 1994.
- Foreyt, WI et al: Experimental
infections of two llamas with the meningeal worm (Parelaphostrongylus tenuis ).
J Zoo Wildl Med 23:339-344;1991.
- Baumgartner, W et al:
Parelaphostrongylosis in llamas. J Am Vet Med Assoc 187:1243-1245;1985.
- Welles, EG et al: Composition
of cerebrospinal fluid in healthy adult llamas. Am J Vet Res 55:1075-1079;1994.
- Dew, TL et al:
Parasite-specific immunoglobulin in the serum and cerebrospinal fluid of
white-tailed deer (Odocoileus virginianus) and goats (Capra hircus) with
experimantally induced parelophostrongylosis. J Zoo Wildl Med 23:281-287;1992.
- Rickard, LG. Parasites. Vet
Clin North Am Food Anim Pract 10:239-247;1994.
- MacDiarmid, SC. Clinical
pharmacology of the female reproductive tract: manipulation of parturition, its
sequeslae and infections. Clinical Pharmacology and Therapeutics Proceedings
71:21-45;1984.
- Kopcha, M et al: Cerebrospinal
nematodiasis in a goat herd. J Am Vet Med Assoc 194:1439-1442;1989.
More Meningeal Worm Information
M-Worm Description by Ruthanne McCaslin, DVM
|
|
Treatment for Meningeal Worm -
August, 2003
David E Anderson, DVM, MS, DACVS
Head and Associate Professor of Farm Animal Surgery
Director, International Camelid Initiative
Ohio State University
College of Veterinary Medicine |
Our current TREATMENT protocol is:
Fenbendazole (Panacur or Safeguard) at 50 mg/kg body weight
orally daily
for 5 days
Flunixin (Banamine) 1 mg/kg body weight subQ, twice daily for 3
days,
once daily for 3 days
Vitamin E supplement 500 to 1000 units orally daily for 14 days
Omeprazole (Gastrogard) 2 to 4 mg/kg orally daily 7 to 10 days
Physical therapy. (Hints
on how to make a sling to raise the llama)
Note:
Ivermectin or Dectomax are good for PREVENTION, not TREATMENT.
Neither of these drugs enters the central nervous system which
is where the worms are in CLINICAL cases. |
This most recent article was sent out in May, 2001, by Dr. David Anderson from Ohio State University.
"This article is from Dr. Cliff Monahan, our parasitologist researching camelids. He says some thought
provoking things! (Cliff Monahan, DVM, PhD; Dept. Veterinary Preventive Medicine; Ohio State University College of Veterinary Medicine)".
"Parelaphostrongylus tenuis is a very real concern in areas of the east where
white-tail deer are prevalent. My talk will focus on the biology of the parasite, the
epidemiology of the disease seen in camelids within the Ohio River Valley, and
control programs that may be more relevant than the monthly treatments presently
employed. These monthly treatments as a preventive program have led to drug
resistance in the nematodes normally infecting several susceptible species. This
highlights the need to have alternatives to the intensive anthelmintic prevention
program used today.
Life Cycle: Parelaphostrongylus tenuis utilizes the white-tail deer as its definitive host
and has an indirect life cycle, meaning there is an obligatory developmental stage in
snails or slugs. The disease CANNOT be passed without the ingestion of an
infected snail or slug. First-stage larvae are passed in the feces of P. tenuis
infected WTD and these must be ingested by gastropods for development into the
infective 3rd stage larvae. Once ingested by snails or slugs, the 1st stage larvae
require warmth to develop, thus the rate of development will depend on the ambient
temperature. Continued cold weather slows development. Next the snail or slug
must be ingested by a susceptible WTD, or in the case of aberrant infections,
ingested by a susceptible sympatric ruminant. Without ingestion of the gastropod
carrying infective 3rd stage larvae, the infection is not transmitted. Many snails
and slugs are consumed inadvertently by grazing or browsing animals, but only during
parts of the year conducive to snails and slugs. Gastropods will be less active during
cold weather, will hibernate during freezing weather, and will estavate during hot, dry
weather.
Clinical cases of meningeal worm affecting camelids have followed a distinct pattern
of disease here at the Ohio State University Veterinary Teaching Hospital --
This pattern may not exist in your area!!!! Two major peaks of disease are seen
by Dave Anderson here at Ohio State, the major peak being Sept/Oct, the second
during Jan/Feb. This implies that there are 2 peak seasons of transmission to
correspond with the peaks of disease. During studies conducted at the Wilds in
southeastern Ohio, we found that there were no snails or slugs present during freezing
temperatures, and the numbers of gastropods increased in the spring as weather
warmed, but it remained moist and relatively cool. The heat and dryness of mid
summer drove the snails and slugs into estavation, then they reappeared when
temperatures cooled again in late summer, early autumn.
Based on the 2 peaks of disease here at Ohio State, the necessity of gastropods for
transmission of P. tenuis and the 2 peaks of gastropods on pasture, we speculate
that the most important times for meningeal worm prophylaxis are these 2 times when
gastropods are most prevalent.
This is relevant here in the Ohio River Valley. YOUR area may have some
variation on the prevalence of gastropods, thus you MUST adapt these
findings to your own area. Further north there may be shorter periods, and
further south longer periods.
These recommendations are made in light of the relative risks of P. tenuis
transmission and the very likely risk of developing drug resistant llama and
alpaca parasites secondary to overuse of ivermectin or other macrocyclic
lactones.
Please pay attention to what I am really saying:
1) Overuse of the avermectins (as I see regularly within the camelid
industry because of meningeal worm prevention programs) is destined to
create more problems than P. tenuis. If these practices create drug
resistant camelid parasites by the monthly use of ivermectin, these
parasites will be resistant to doramectin and moxidectin as well. The
industry will be better served to avoid this eventuality.
2) Due to the seasonality of snails and slugs in our area, I recommend
that camelid owners consider using drugs for prophylaxis during the peak
risk timepoints, and I recommend that they do not use ivermectin year
round for this purpose. This program does not give you 100% protection,
but I can tell you all that the creation of drug resistant camelid parasites will
be much more a problem.
3) My theoretical position is that camelid owners can use a
long-acting macrocyclic lactone, such as doramectin, and this will reduce
the number of treatments needed for protection. By reducing the overall
number of treatments, you will delay or avoid the development of drug
resistance.
IF YOU USE THESE LONG-ACTING DRUGS IN THE SAME
FASHION THAT YOU USE IVERMECTIN, YOU DEFEAT THE
PURPOSE AND ENHANCE THE DEVELOPMENT OF DRUG
RESISTANCE!!!
4) Theoretically, you could inject an animal on May 1st and this would
kill any migrating larvae on-board since April 1st. As our preliminary
research shows us that there are not many infected snails or slugs present
in April, this risk seems reasonable. The long-acting effect of doramectin
in cattle kill any infective larvae ingested on pasture for approximately 28
days. I am speculating that the same level of protection will be provided to
camelids, thus you do not need to treat again for almost 60 days.
Why 60 days? Theoretically, no ingested larvae will survive while the
long-acting drugs are at these levels. After the 28 days of protection have
elapsed, you should have ~ 30 day period when any ingested larvae are
migrating and susceptible to treatments. I admit this must be verified with
experiments in camelids, but this is a rational expectation. So, your
injection on May 1st is good until May 29th based on residual activity, then
you may add another 30 days before you need to treat again. This means
1 treatment every 60 days instead of every 21 days that I hear regularly."
Another Article From Dr. David Anderson, DVM - Ohio State University:
"Meningeal worm (Parelaphostrongylus tenuis) represents a significantly different problem to llamas.
These worm larvae are passed through the feces of deer (natural reservoir), are consumed by snails, and
then are consumed by llamas and alpacas. Llamas are not the normal host for these worms and they
perform "aberrant migration". During this migration, they may travel into the spinal cord and cause
significant harm to the host - even causing lethal consequences. Fencing deer out of the pasture is not
enough and chemicals to kill snails cause environmental residues that may be harmful and are of limited
efficacy. Therefore, most prevention against meningeal worm larval infection is aimed at killing the
larvae during their migration, but prior to entry into the spinal cord. This requires a de-worming
frequency of at least every 4 to 6 weeks at least during the high risk periods of the year (April-May
through November-December in Ohio). The most efficacious anthelmintics for protection against
meningeal worm have been ivermectin (1 cc of 1% ivermectin per 100 pounds body weight, injected
under the skin, every 4 to 6 weeks) or fenbendazole (4.5 cc of 10 % fenbendazole per 100 pounds body
weight, given orally, once daily for 3 to 5 days)."
From Dr. Stephen R. Purdy, DVM - Chester, Vermont:
Meningeal Worm - Diagnosis, Treatment, & Prevention
From Dr. David Anderson, DVM - Ohio State University
Meningeal Worm - Infection In Llamas & Alpacas
Meningeal Worm - Infection In The Ohio River Valley
Meningeal - Prevention Diagnosis, & Treatment
TREATMENTS for M-Worm
TREATMENT PROTOCOL
For many years, the protocol known as the “Buckeye Blast” developed by Dr.
David Anderson while at Ohio State University was the recommended
treatment for meningeal worm infection. Today, this protocol is still
being used, although time and experience have modified it somewhat.
The most critical ingredient is fenbendazole (Safeguard), which kills the
parasites present in the CNS. The recommended dose is 50 mg per kilogram
bodyweight for five days. Although lower doses (20 mg/kg) have also been
shown to be effective in many cases, owners may wish to err on the side of
caution given how often much of a dose ends up on the animal, rather than
in it.
Flunixin (Banamine) is recommended in addition to the fenbendazole as an
anti-inflammatory agent. Much of the damage caused by the parasites is
created by inflammation and swelling where they have been active in the
nervous system, and Banamine helps to mitigate these issues. The
recommended dose is 1 mg per kilogram bodyweight, twice daily for three
days, then once daily for an additional three days.
Although omeprazole (Gastrogard) was previously recommended due to the
possible ulcerative properties of Banamine, it has been shown that a) oral
Gastrogard is not effective in camelids [Poulsen, 2005] and b) Banamine is
unlikely to be ulcerative in this time frame [Evans, 2005]. Injectable
avermectins are not recommended as part of the treatment protocol, as they
cause further damage if they cross the blood-brain barrier [Van Amstel, et
al., 2009].
Vitamins are also often included in the treatment protocol, as many serve
to help protect and/or promote regrowth of the nerves damaged by the
parasite. Thiamine, vitamin E and additional B complex vitamins can all be
administered to the alpaca under treatment. Vitamin E is fat soluble and
care needs to be taken not to overdose, but the B vitamins are water
soluble, and any excess is excreted by the alpaca in urine.
Recently, the methyl form of vitamin B12 (methylcobalamin)
has been shown to be effective in promoting nerve regeneration due to
injury, diabetic neuropathy and other causes [Yagihashi, 1982; Watanabe,
1994; Yamazake, 1994; Jacobs, 2009]. This vitamin may be beneficial for
meningeal worm survivors. No information concerning appropriate dosing for
alpacas currently exists, but extrapolating from information on the use of
this vitamin in dogs and horses indicates that 2 mg per kilogram
bodyweight would not appear to be unreasonable.
(author unknown) |
|
Treatment for Meningeal Worm -
August, 2003
David E Anderson, DVM, MS, DACVS
Head and Associate Professor of Farm Animal Surgery
Director, International Camelid Initiative
Ohio State University
College of Veterinary Medicine |
Our current TREATMENT protocol is:
Fenbendazole (Panacur or Safeguard) at 50 mg/kg body weight
orally daily
for 5 days
Flunixin (Banamine) 1 mg/kg body weight subQ, twice daily for 3
days,
once daily for 3 days
Vitamin E supplement 500 to 1000 units orally daily for 14 days
Omeprazole (Gastrogard) 2 to 4 mg/kg orally daily 7 to 10 days
Physical therapy. (Hints
on how to make a sling to raise the llama)
Note:
Ivermectin or Dectomax are good for PREVENTION, not TREATMENT.
Neither of these drugs enters the central nervous system which
is where the worms are in CLINICAL cases. |
|
Treatment for Meningeal Worm -
From Dr. Norman Evans Field
Manual - June 2003
"This manual expresses my current opinions as of June 20, 2003.")
Treatment for m-worm as Ivomac or Dectomax at 1 cc.25 lb body weight SQ
every 24 hours for 3 times along w/ Banamine at 1 cc/100 lb every 24 hours
for 3 times. This treatment protocol has shown
some promise when used early."
"When used early, 90% DMSO at 30 cc/100 lbs body weight diluted in 1000 cc fluids and administered IV for 3
days has shown nice results."
|
|
 |
 |
 |
Return To
Vet Info |
Return To
Llama Management |
Return To
Shagbark Ridge Llamas |
|

