|
What Is It?
The
liver is one of the biggest and most important organs in the body and keeps the
body's metabolism and function in balance. On a biochemical level, it is
responsible for many essential processes. The failure of any of these
biochemical functions could lead to the death of the animal. The liver can
be affected by diet, infection, drugs, and other toxic chemicals.
From Infovets.com:
"Some of the main functions
of the liver include proper handling of carbohydrates (sugars, starches), lipids
(fats, cholesterol, bile acids), and proteins. Some of the important proteins
produced by the liver include blood clotting factors, urea (used by the kidney
to help with the body’s water preservation), and albumin (the main protein in
the blood which helps maintain the proper fluid volume in the heart and blood
vessels). Essential vitamins and minerals are produced, stored, or altered by
the liver for proper use in the body. The liver aids in the functions of the
immune system, the endocrine system, and in maintaining healthy blood cells.
Bile acids are produced and stored in the liver and gallbladder, and are used in
the digestive tract in the breakdown of food.
One very important function of the liver is its role as the body’s filter from
the digestive tract. The entire digestive tract contains bacteria and food in
various stages of breakdown. Nutrients are absorbed into the blood through tiny
capillaries from the stomach and intestines. Blood from the digestive tract may
be thought of as "dirty," since it is so close to a source of contamination—
millions of bacteria and possibly harmful substances taken into the body through
the mouth. From here, the blood enters the liver’s portal blood system, where
the liver can "detoxify" any harmful substances and acts as the first line of
defense against invading bacteria.
The liver has a tremendous ability for regeneration
and functional reserve. It is able to tolerate injury and insult better than
most organs without failing (although sickness may be very apparent in the body)
and can repair itself remarkably well following damage. These are wonderful
features of this vital organ, but they can also make it difficult to recognize
when a serious disease of the liver is present."
Liver Disease in Camelids (4-14-02)
David E Anderson, D.V.M., MS, Diplomate ACVS
College of Veterinary Medicine
The Ohio State University, Columbus, Ohio
Liver disease has not been widely recognized in camelids (alpacas and llamas).
Although fatty liver disease is often discussed or observed during necropsy
examination, primary liver diseases have been infrequently diagnosed. Recent
clinical data suggest that the cause of fatty liver disease may have been
overlooked in many camelids. Because camelids are not used for intensive meat or
milk production in North America and they usually are not overworked by riding
or carting, theories postulated for onset of liver disease in other livestock
species may not apply to camelids. Investigations of camelid disease is in its
infancy in the USA, and I expect that significant breakthroughs are on the
horizon. We have begun investigations at Ohio State University to attempt to
better determine the response of camelids to disease and stress.
HISTORICAL INFORMATION
I often find evidence of liver disease in camelids while working-up a case of
what might be termed "Sick Camelid Syndrome" or SCS. SCS refers to a camelid
that demonstrates depression, lethargy, increased periods of recumbency, and
decreased appetite. Occasionally diarrhea or abdominal discomfort are observed.
The results of physical examination fail to reveal any abnormalities except that
the animal may be more tender to palpation of the abdomen than normal. In my
opinion, laboratory testing of these animals is obligatory because of the stoic
nature of these patients. A camelid may not show clinical signs of severe
disease in a desperate attempt not to be singled out from the herd as a
"weakened" animal by potential predators. The most important information to be
gained by veterinarians from the owner is a detailed account of the animals
recent lifestyle. Any changes should be discussed (nutrition, herd mates, feed
bags, traveling to shows / sales / new farm, breeding activity, importation and
quarantine, de-worming and vaccines or lack thereof, obesity, heat stress,
emaciation, etc). Most cases of liver disease are found to be caused by or
exacerbated by stress.
PHYSICAL EXAMINATION AND CLINICAL SIGNS
A thorough physical examination is required to rule-out common causes of illness
in camelids: parasites, GI disturbance, C3 ulcer, starvation or malnutrition,
social hierarchy, dental problems (tooth root abscess, malocclusion, etc),
neurologic disease (meningeal worm, trauma, infection), pneumonia, etc. Rectal
temperature, heart rate and rhythm, respiratory rate and pattern, C1 motility
frequency and pattern, fecal consistency / color, urine color / clarity,
peripheral lymph node palpation, and oral examination are the minimum veterinary
data base. Clinical signs will dictate additional diagnostic tests, but most
suffer from Sick Camelid Syndrome and, therefore, I routinely perform a complete
blood cell count and serum biochemistry profile. I have seen many camelids
suffering from severe liver disease demonstrate clinical signs of acute
abdominal pain. These patients must be accurately differentiated from surgical
colic because the stress of surgery and general anesthesia is extremely
detrimental to patients with liver disease.
I have a liver and kidney profile run which includes SDH, GGT, AST, CPK,
triglycerides, cholesterol, Cr, and BUN. SDH and GGT are cellular enzymes that
provide a reflection of the severity of liver damage. AST and CPK are cellular
enzymes that reflect muscle injury and allow some assessment for how long and
how often the camelid has been lying down. Triglyceride and cholesterol are
components of fat and provide an assessment of how much lipid mobilization is
occurring. Creatinine and BUN are products of protein metabolism that are
excreted by the kidneys and allow evaluation of general kidney function. I have
found that rising BUN and Cr despite supportive therapy is a poor prognostic
indicator. Electrolytes and bicarbonate status are evaluated because worsening
acidosis, increasing GGT, and increasing Cr despite supportive care are
indicators of a grave prognosis for survival.
Although viral and bacterial hepatitis are occasionally diagnosed in camelids,
secondary bacterial infection is most common. Of particular concern is invasion
of clostridia. Therefore, the complete blood cell count is evaluated for
evidence of bacterial infection and the PCV and total protein examined. I have
found that a rising PCV in the presence of a falling T.P. is a grave prognostic
indicator. Also, I use blood immunoglobulin concentration as a screening tool to
evaluate immune system status.
ETIOLOGIC INVESTIGATIONS: DIAGNOSIS?
Diagnosis of the cause of liver disease in camelids can be an exercise in
frustration. Histopathology (microscopic examination of liver tissue by means of
liver biopsy) usually is not specific: hepatic lipidosis, biliary hyperplasia,
lymphocytic plasmacytic hepatitis are common findings. Occasionally
cholangiohepatitis (infection of the bile ducts) or cholestasis (obstruction to
bile flow) are diagnosed from biopsy. Although histopathology often does not
provide a definitive diagnosis, the information gained is well worth the effort.
Because few specific liver diseases have been described for camelids,
differential diagnoses should be broad in range: metabolic (e.g., fatty liver,
cirrhosis), parasitic (e.g., liver flukes), toxic (e.g., mycotoxin, endotoxin,
clostridium spp), bacterial (e.g., Salmonella spp, Clostridial spp, E coli),
viral (e.g., adenovirus), fungal (e.g., Coccidioides imitis), and tumors or
cancer (e.g., adenocarcinoma). I routinely perform ultrasound guided
percutaneous liver biopsy and obtain samples for histopathology, virology, and
bacteriology. Recently, a picornavirus has been identified at Cornell University
(Dr. Susan Stehman and colleagues) which appears to cause pancreatitis and
destruction of insulin producing islet cells. The result of this is insulin
dependent diabetes mellitus which can lead to ketoacidosis and death.
TREATMENT STRATEGY
Treatment is directed at supportive care unless a more specific diagnosis can be
determined. Antibiotics, anti-inflammatory drugs, fluid therapy (I prefer oral
fluids when possible), glucose supplementation, and pain therapy are useful for
treatment of severe liver disease. Insulin therapy must be used judiciously so
that a harmful decrease in blood glucose does not occur. When used, intravenous
fluids must be administered cautiously because camelids readily develop low
blood protein with liver disease. Anti-ulcer prophylaxis is critical to prevent
clostridial overgrowth. I prefer to use omiprazole because this drug is more
potent than Tagamet. Clostridial antitoxins or vaccination may be useful to
bolster immunity.
The most critical factor for treatment of camelids with liver disease is to keep
them eating. If appetite is suppressed, transfaunation (administration of the
rumen fluid from a cow into the stomach) is a potent appetite stimulant. Other
options include bacterial supplements products (such as probios), yogurt,
B-complex vitamins, use of a companion animal, and offering a variety of feeds
including frequent grazing. Camelids may lay down and refuse to get up if
isolated in a stall. These animals should be walked, grazed, and a companion
animal kept with them to prevent this cycle from starting. I have had the most
success reversing liver disease in camelids by increasing the energy density of
the diet (originally suggested by Dr. Norman Evans). I recommend that a glucose
enriched electrolyte water be available at all times. Calf electrolyte solutions
are excellent, but many of my clients have used Gator aid and similar products.
Energy density may be increased in the diet by supplementing sweetfeed, dried
molasses, syrup, etc. These supplements should be made available until liver
enzymes have returned to normal.
PREVENTION OF LIVER DISEASE
I have performed extensive investigations into copper toxicity, mycotoxin
contamination, parasite infestation, water source contamination, and have found
that most cases of liver disease can not be readily explained. Therefore,
recommendations for prevention are difficult. Probably, the most significant
factor in the prevention of liver disease is to prevent sustained stress. I have
found that the most severe cases of liver disease have been in camelids
suffering severe, long-term stress. An example would be a llama or alpaca that
is acquired in Peru, moved to a quarantine station in Peru, examined and treated
several times by veterinarians and animal handlers, moved to a quarantine
station in the United States, moved to a farm for sale, sold at auction, moved
to the farm of final destination, entered into a new herd to establish a new
social hierarchy, and, finally entered into the breeding pool. These events
occur over approximately 8 to 12 months. Hepatic lipidosis is the most common
consequence even in relatively thin animals. A common misconception is that
fatty liver disease is a disease of overweight camelids. I have found that these
are the exception, not the rule. To prevent the development of these adverse
effects, the environment in which the animals are moved should be as free from
stress as possible, animals should be vaccinated with 7-way or 8-way clostridial
vaccines, high quality grass hay or grass should be available at all times, and
a trace mineral mix should be available.
When I see fatty liver disease occurring in domestic camelids, I believe that
the nutritional program should be intensively investigated. If the feed source
has changed recently, a feed analysis is indicated to determine if the feed is
low in digestible energy. The best indicator of the adequacy of the diet is to
analyze mineral content in liver biopsies. Research done at Ohio State
University investigated the effects of repeated liver biopsies. Despite
performing biopsies at weekly intervals, no adverse effects were observed (in
fact the animals gained an average of 5 pounds!). However, I recommend whole
blood analysis of minerals if the animal in question is pregnant. Research done
at Ohio State University has documented that Alpacas are quite resistant to
fumonisin (a type of mycotoxin) intoxication. We fed up to 75 parts per million
of fumonisin for up to 30 days with no adverse effects on the serum
biochemistry, complete blood cell counts, and liver tissues. We are continuing
to investigate liver disease and nutrition in camelids at Ohio State University.
Research is critical to determine the cause of liver disease in these animals
because the initial cause is usually past by the time your veterinarian gets
involved in the case. Some very exciting research is being done in similar areas
at Cornell University, Oregon State University, Auburn University, and Colorado
State University. Hopefully, we will have better answers for you in coming
years!
 
Action Plan for Camelids (Alpacas, Llamas)
Affected with
Acute Death Associated with Liver Disease
David E Anderson, D.V.M., MS, Diplomate ACVS
College of Veterinary Medicine
The Ohio State University, Columbus, Ohio
CONTROL AND SURVEILLANCE
- Do not change social
structure of groups. Leave animals that have established a social order
together. Stop all new activity on farm (e.g. show fitting and testing,
re-grouping for sale, introduction of new animals, removal of animals).
- Evaluate all feed and
water sources. Remove any suspect hay or grain sources (e.g. molded, spoiled,
etc). Clean and sterilize any water containers that appear to contain algae or
are not clean. Inspect all water sources for evidence of dead animals, run
off, etc.
- Obtain samples from all
feed and water sources. Have hay and feed analyzed for nutritional values and
trace mineral content. Have hay and feed analyzed for aflatoxin and fumonisin
mycotoxin. Have water analyzed for mineral content, pH, and bacterial inoculum.
- Perform liver mineral
analysis and intestinal cultures on all animals that die. Perform trace
mineral panel and viral profiles on all animals that have blood drawn for any
other purpose.
- Check CBC and serum
biochemistry profile on all symptomatic animals to guide additional treatment
decisions. May elect to check serum biochemistry profile (the most diagnostic
and prognostic testing tool) on all asymptomatic animals (optional - concern
is additional stress).
- Perform complete
post-mortem examination of all animals that die. Save necropsy specimens from
heart, liver, lung, kidney, C1 content, urine, and aqueous humor for future
toxicology as indicated by histopathology.
TREATMENT RECOMMENDATIONS
- Provide low stress
environment - see comments above.
- Provide source of readily
available carbohydrates. These include but are not limited to glucose and
electrolyte enriched water, dried molasses, sweet feed, and high quality hay.
Continue to provide plain clean water.
- Provide top dress in feed
to include vitamin, mineral, bacterial / yeast, and methionine supplement.
- Treat animals
symptomatically based on appearance, physical examination, and laboratory test
results. Antibiotic, anti-inflammatory, and ulcer therapy are administered on
a case-by-case basis. I have had the best success with sodium ceftiofur
(Naxcel, 2.2 mg/kg, s.c., q24hr), banamine ( 1 mg/kg, s.c., q12hrs), and
omiprazole (prilosec, 1 mg/kg, p.o., q24 hrs). Avoid any use of steroids.
PREVENTION RECOMMENDATIONS
- Maintain low stress
environment (sun, shade, ventilation).
- Ensure proper Clostridial
vaccination protocol.
- Ensure appropriate
parasite control strategies.
- Minimize movement and
re-grouping of animals. 5. Make long range plans for animal grouping
organization so that repeated changes in group social structure can be avoided
(e.g. breeding and sale activities).
 
Views on Fatty Liver Disease
David E. Anderson, DVM, MS, Diplomate ACVS,
Bradford B. Smith, DVM, PhD
Susan Tornquist, DVM, PhD
Robert Van Saun, DVM, PhD
Few diseases that can strike alpacas (and
llamas) are as insidious as fatty liver disease. An alpaca farm that has all
the good management signs of a tidy, well-run operation can lose animals to this
disease as readily as a casually run operation scorned by neighboring breeders.
Animals in good body condition can be struck by
it, though often animals with low body scores are susceptible. The mechanisms
setting the disease into motion aren’t entirely understood, other than that
diets low in protein and other essential elements create risk. The disease
doesn’t normally occur until after a significant amount of time on a deficient
diet. Often once one animal is stricken others follow. Any breeder experiencing
a death to fatty liver disease should assume that other animals experiencing the
same diet and exposure to stressors are at risk and consult a veterinarian.
The disease often appears in those alpacas in a
population experiencing the most stress. A recent outbreak occurred in a group
of lactating females a week after the onset of cold and wet weather. Animals not
lactating in the same herd suffered no ill effects. Apparently the lactating
females’ bodies were working overtime to supply milk to their crias. Later
testing indicated that the rye hay they were fed contained only about 8 percent
protein. Also, the animals were receiving a daily grain/pellet supplement.
Shortly after the onset of cold weather one
lactating female slowly quit eating and became sluggish. Her symptoms grew more
severe. A slight yellow cast in skin near her eyes and in her mouth appeared,
indicating poor liver function, which blood test also verified. Unfortunately,
the focus on strategies to cope with her problems began too late. The animal
died in three days, at which time a second animal showed the same symptoms and
died two days later.
A rapid analysis of feed was conducted. Herd
owners were shocked to learn that the hay in use contained only 8 percent
protein. Earlier shipments from the same supplier had always contained 12 to 15
percent protein. The lesson learned is that even with the same supplier and the
same hay type, there are no guarantees feed will have the same nutritional
quality from year to year. The hay in question looked very edible and was
readily consumed by animals exposed to it. The grower had assured the alpaca
breeder that the hay was "good quality and like past years." It turned out that
because of floods that tore away topsoil, the hay was not properly fertilized
and subpar.
Other alpaca ranches also using the hay were
contacted. None had lactating females, but one ranch attributed an animal’s
death to fatty liver disease.
All involved farms abandoned their hay and
absorbed the loss. All alpacas on several different ranches were given a richer
hay (50-50 orchard-alfalfa mix) that tested at about 15 percent protein. Before
the arrival of the new hay, monitoring of enzyme activities indicated that other
animals had impaired liver functions and were on the verge of succumbing to the
disease. After three weeks on the new diet all of the affected animals’ liver
functions returned to normal.
In part the problem in the herd described above was exasperated by research and
rhetoric in llama nutrition that emphasized the problem of obesity in llama
herds. In the llama business the emphasis on a nutritionally balanced but
not-rich diet to avoid obesity may not be as applicable to alpacas. In fact,
several leading camelid veterinarians feel that alpacas may indeed thrive on a
richer diet than llamas do. The two species have different dietary preferences
in South America. One California breeder who has llamas and alpacas found that
when he maintained both on the same diet (14 to 20 percent protein), for the
most part alpacas maintained optimum body condition and llamas became obese.
Could it be the two species have somewhat different dietary needs? At this time
these observations are anecdotal, but research occurring at Oregon State
University and Ohio State University will likely lend more understanding to the
dietary needs of alpacas and how they may differ from those of llamas. —Editor
 
Hepatic Lipidosis in the Camelid: A
Different Perspective
Bradford B. Smith, DVM, PhD
Susan Tornquist, DVM, PhD
Robert Van Saun, DVM, PhD
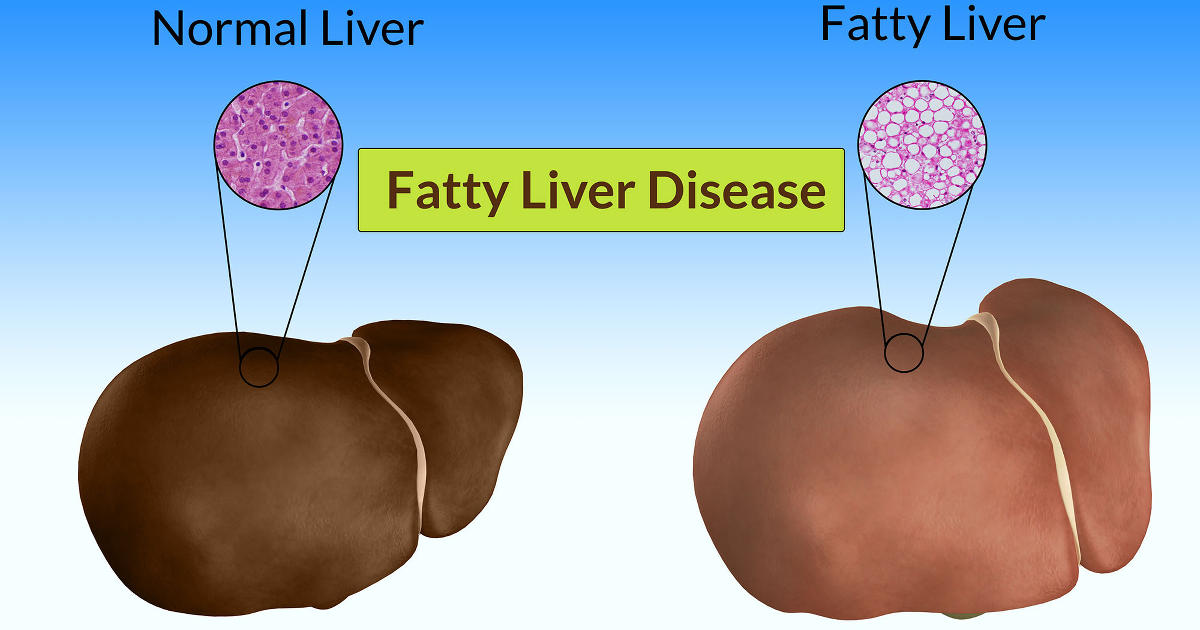
What is hepatic lipidosis?
As they say in the advertising field, the name
says it all. Hepatic means liver; lipidosis means an accumulation of lipids or
fats. Functionally it is a condition in which the liver has accumulated a large
quantity of fat within the cells.
If you microscopically examine a normal liver,
you will see all the usual components of a cell, including such structures
(organelles) as the nucleus, mitochondria, lysosomes, and an occasional lipid
(fat) droplet. The liver is one of the most metabolically active tissues in the
body and plays a central role in fat metabolism, converting the lipids from one
form to another as needed.
Hepatic lipidosis occurs when the amount of fat
accumulating in the liver becomes excessive, compromising liver function. In
this case, most of the cell becomes filled with fat droplets and the normal
functions of the cell are impaired. As a consequence, the normal role of the
liver in clearing toxins and other waste products is impaired, the byproducts
accumulate in the blood, and the animal dies as a result of liver failure.
Understanding why apparently normal animals can
have no evidence of problems and then die within a period of 3 to 4 days from
acute liver failure is perplexing; even more perplexing is the apparent absence
of other identifiable problems in most cases.
The first step in sorting out this mystery has
been to take a retrospective look at what we know about the problem. To this end
Dr. Sue Tornquist, head of Clinical Pathology at Oregon State University (OSU),
called up the medical records of twenty-six llamas and alpacas with a
histopathologic diagnosis (that is, the diagnosis was based upon a microscopic
examination of the tissues and not just a gross anatomical evaluation) of
hepatic lipidosis during the period from 1991 to 1997. Of the 771 llama and
alpaca submissions to the OSU Veterinary Diagnostic Laboratory during this
period, hepatic lipidosis was identified in twenty-six, or 3.4 percent, of the
cases. When the twenty-six cases were more closely examined, the following
observations emerged:
Sex: Twenty-two (or 88 percent of the group) were
females, a significantly higher percentage of females than in the original 771
submissions.
Physiologic state: Twelve of the affected animals
were known to be pregnant (52 percent of the females), and ten were known to be
lactating (43 percent of the females).
Age: Ages of animals with hepatic lipidosis
ranged from 5 months to 18 years of age, with a mean age and standard deviation
of 7.1 ±4.6 years (66 percent of the population). In contrast, only 14 percent
of the reference population in which ages were known were between 6 and 10 years
of age.
Presenting signs: Although thorough history,
physical exam findings, and laboratory data were not available for all cases,
the most common factors were
• A history of recent severe anorexia or weight
loss in 58 percent of the cases
• Neurologic signs including incoordination,
blindness, and head pressing in 27 percent of the animals
Other changes were either agonal or present in
only a few animals.
Interpretation
This retrospective work suggested that hepatic
lipidosis was primarily a disease of middle-aged llamas and alpacas, with a
substantially higher incidence in females, particularly females with a high
metabolic demand such as late-term pregnancy and/or heavy lactation. These
observations, coupled with the lack of other consistent histopathologic changes
or evidence of bacterial or viral infections, has suggested that the problem may
be primarily nutritional and/or metabolic in nature.
Clinical Cases
Retrospective studies tell only a portion of the
story and don’t provide good information about the development of the problem.
Herd outbreaks in the past several years have given us a better understanding of
the dynamics of the problem within a herd, such as timeframes for the
development of the problems, clinical changes, and alterations in blood
parameters. To address this point, the following is a brief description of four
herd outbreaks we have seen in the past couple of years.
Case 1: Four llamas developed the problem within
a 4-week period. Although the quality of the forage was good, the volume of feed
being provided was very low. No toxins
were identified. Other than insufficient
feed, no additional stress factors were
identified.
Case 2: Three female llamas developed hepatic
lipidosis within a few weeks. All of the animals were lactating, and the forage
quality was high. The onset of the problem coincided with a shift to very high
temperatures. Changes in the weather and social hierarchy were identified as
possible factors leading to decreased forage consumption.
Case 3: Three lactating female alpacas died
within a short period of time. The onset appears to have been correlated with a
shift to cold weather. The forage quality appears to have been marginal though
supplement had been offered to compensate. Two animals were in good condition at
onset of disease.
Case 4: A group of seven llamas died within a
3-week period. This was the only outbreak in which most of the animals were
males. Unlike the females in the other cases, which for the most part were over
conditioned, in this situation the pastures and hay were of poor to very poor
quality and most of the animals were moderately to severely under conditioned.
Interpretations
All these cases support our view that we are primarily dealing with a metabolic
disease with underlying nutritional problems, specifically that the animals are
in a condition of very high energy demand and are not able to meet this need in
an appropriate manner. As a result of the increased energy needs, more and more
fat is mobilized. For reasons that are not clearly understood, the liver is
unable to handle the increased demand for energy and responds by inappropriately
increasing the amount of fat stored in the liver—ultimately resulting in liver
failure.
A couple of important caveats about this
interpretation:
• Inappropriate energy and/or protein metabolism
as the cause of the problem is a theory—not a fact—and needs to be carefully
examined.
• In all cases, the problem appears to have a
stress component, such as changes in social conditions, dramatic changes in the
weather, or restricted food availability. While stress appears to be a
triggering device, its role in the development of the problem is unclear.
It was against this background that Susan
Tornquist, Bob Van Saun, and I developed a research protocol to examine the
metabolic basis for hepatic lipidosis in the llama. We submitted the proposal to
the Morris Animal Foundation, where it was reviewed, evaluated, ranked, and
funded. (We wish to thank Andy and Cheryl Tillman for funding this project
through the Morris Animal Foundation.) We are currently running a series of
studies to establish how the disease develops and to characterize the clinical
and biochemical changes so that the condition can be identified at a stage early
enough for treatment. The project at Oregon State University has so far produced
some fascinating results and generated a much better understanding of the
condition.
Conclusions
Hepatic lipidosis is becoming a serious problem.
While we still don’t have the answers, work at Oregon State University is an
important first step in finding a solution. If you have had confirmed camelid
deaths caused by hepatic lipidosis, we would be very interested in hearing from
your veterinarians about these cases. These talks have been very useful in
helping us get a more detailed and complete picture of the problem under field
conditions and a better understanding of the underlying factors involved in the
development of the condition. Confidentiality is maintained at all times.
Contact us at College of Veterinary Medicine, Oregon State University, 105
Magruder Hall, Corvallis, OR 97331-4802, fax: 541-737-0502.
Copyright © 1998 by Brad Smith, Susan Tornquist,
and Robert Van Saun.
About the Authors
David E. Anderson, DVM, MS Diplomate ACVS, is an
assistant professor of farm animal medicine, College of Veterinary Medicine,
Ohio State University, Columbus. He has been practicing medicine and surgery and
performing research with llamas and alpacas for 8 years, focusing on
reproduction, nutrition, and metabolic disease, all of which are closely
interrelated. His current six research projects on alpacas and llamas are
directed to developing immediate applications "on the farm."
Brad Smith, DVM, PhD: Oregon State University
Susan Tornquist, DVM, PhD, ACVCP, is the head of
clinical pathology at Oregon State University. She is currently working on two
Morris Animal Foundation grants dealing with camelid health issues.
Bob Van Saun, DVM, PhD, ACT, ACVN, is a
double-board-certified faculty member at Oregon State University with an
interest in nutrition and reproduction. He has been working with llamas and
alpacas for the last 5 years and is involved in several studies. |